Tumor Mismatch Repair Immunohistochemistry as a Prescreen for Lynch Syndrome-Associated Ovarian Cancer
Ovarian cancer is the seventh most common malignancy worldwide and the most deadly gynecologic cancer.1 It has been termed the silent killer because its presenting symptoms are often mistaken for other benign conditions and is difficult to detect early through screening. Epithelial ovarian cancer is one of the most common hereditary malignancies predominantly due to pathogenic variants in the BRCA1 and BRCA2 genes. The other leading hereditary cause is Lynch syndrome (LS), an inherited mismatch repair (MMR) deficiency due to pathogenic variants affecting one of four MMR genes, MLH1, MSH2, MSH6, and PMS2, which are involved in DNA repair.2 About 1 in 280 individuals carries a pathogenic variant in a MMR gene.3 In addition to being associated with ovarian cancer, LS also increases the risk of colon cancer, endometrial cancer, and several other cancers. Identifying those with LS allows for increased surveillance and early cancer detection as well as surgical strategies to lower the risk of cancer.
Since the discovery of the MMR genes in the early 1990s, many methods have been used to try and identify those at risk for LS. The Amsterdam criteria, which were developed in 1991, require a strong family history of colorectal cancer for an individual to be identified as being at risk and has a sensitivity of 61%.4 Even the revised Amsterdam criteria, which added additional LS tumors, such as endometrial and ovarian cancer, has added little to its detection rate or sensitivity (72% sensitivity).4 Capitalizing on the fact that LS is associated with MMR deficiency, both microsatellite instability (MSI) testing and immunohistochemical analyses (IHC) have become valuable diagnostic strategies for LS and are often used as a prescreen to determine when germline testing is indicated. Most of the data on the utility of MSI and IHC testing; however, has involved colon and endometrial cancers. Few studies have looked at the success of this prescreen in ovarian cancer, which as stated previously is a component tumor of LS and requires surgical management to reduce risk.
Study and Methods
To evaluate the utility of tumor MMR in ovarian cancer, Crosbie et al performed tumor testing with IHC for MMR in women, referred from 2000 to 2020 to the regional genetics department in Manchester, with ovarian cancer of any histological subtype diagnosed prior to age 35 and/or with a suggestive personal or family history of LS cancers.5 Where indicated, MLH1 promoter methylation testing was followed by germline testing for LS.
· IHC for the four MMR proteins was performed in the clinical pathology laboratory and tumor epithelial MMR expression was scored.
· Reflex MLH1 promoter methylation testing was performed on tumors showing loss of MLH1 expression on IHC.
· MSI analysis included five mononucleotide-repeat markers. MSI status was reported as microsatellite stable (MSS) where all 5 mononucleotide loci between tumor and matched normal tissue were identical; MSI-low (MSI-L) where there was discordance in one mononucleotide locus and MSI-high (MSI-H) where two or more mononucleotide loci were discordant.
· Germline testing of the four MMR genes was performed on blood samples and utilized Sanger sequencing as well as copy number analysis to detect large genomic rearrangements.
Results
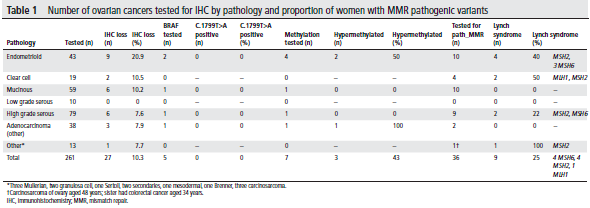
In total, 261 women with ovarian cancer underwent an IHC prescreen for LS (Table 1). Overall, 27 (10.3%) tumors showed MMR deficiency by IHC. Three of 7 with MLH1 loss showed MLH1 promoter hypermethylation, and 18 of the remaining 24 underwent germline testing for LS. An additional 15 women with MMR proficient tumors underwent germline testing due to a strong family history of LS cancers. Pathogenic variants were identified in 9/33 (27%) women who underwent germline testing with a median age of 48 years. Most were of endometrioid histological subtype.
The availability of targeted therapies has led to routine somatic and/or germline BRCA1/2 testing of women with ovarian cancer to determine the appropriateness for PARP inhibitor therapy and clinical trial enrollment. Given that the cumulative risk of ovarian cancer in LS is similar to that associated with BRCA2, testing women with epithelial ovarian cancer for both BRCA1/2 and LS is appropriate, particularly in the era of panel testing. This approach may reduce the need to prescreen ovarian cancer patients for LS. However, as this study suggests, tumor MMR by IHC is a useful prescreen for germline testing in women with ovarian cancer with personal or family histories suggestive of LS and could be of value as a first step in the testing process.
Should you have any questions or would like further information, please contact Nancie Petrucelli at (313) 576-8704/[email protected].
References:
1Zhang Y, Luo G, Li M, Guo P, Xiao Y, Ji H, Hao Y. Global patterns and trends in ovarian cancer incidence: age, period and birth cohort analysis. BMC Cancer 2019;19:984.
2 Ryan NAJ, Glaire MA, Blake D, Cabrera-Dandy M, Evans DG, Crosbie EJ. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med 2019;21:2167–80.
3Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, Buchanan DD, Clendenning M, Rosty C, Ahnen DJ, Thibodeau SN, Casey G, Gallinger S, Le Marchand L, Haile RW, Potter JD, Zheng Y, Lindor NM, Newcomb PA, Hopper JL, MacInnis RJ. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev 2017;26:404–12.
4Syngal S, Fox EA, Eng C, et al Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations inMSH2 and MLH1 Journal of Medical Genetics 2000;37:641-645.
5Crosbie EJ, Ryan NAJ, McVey RJ, Lalloo F, Bowers N, Green K, Woodward ER, Clancy T, Bolton J, Wallace AJ, McMahon RF, Evans DG. Assessment of mismatch repair deficiency in ovarian cancer. J Med Genet. 2021 Oct;58(10):687-691.
back to blog